Finding a patient’s recommended dosage
OLPRUVA® (sodium phenylbutyrate) for oral suspension is taken 3 to 6 times daily with food.1 The recommended dosage of OLPRUVA for patients with urea cycle disorders is 9.9-13 g/m2/day orally.1 Round each individual dose of OLPRUVA to the nearest available dosage strength.
- Divide the calculated total daily dose into 3 to 6 doses and take with food1
- Maximum dosage is 20 grams per day
- Combine OLPRUVA with dietary protein restriction and, in some cases, amino acid supplementation (eg, essential amino acids, arginine, citrulline, and protein-free calorie supplements)
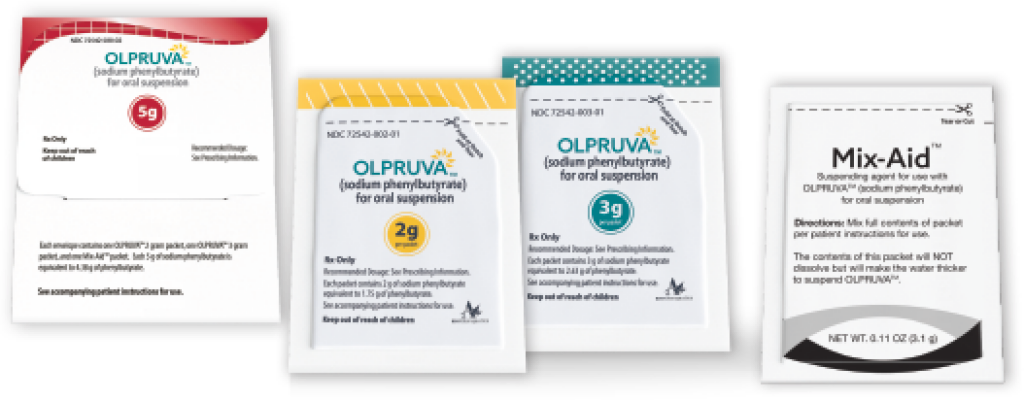
Each kit contains 90 pocket-sized, premeasured dosing envelopes. The 4-g dosage kit is shown as an example. OLPRUVA is available in strengths of 2 g, 3 g, 4 g, 5 g, 6 g, and 6.67 g.
Dosing calculator
Find the recommended dosing for your UCD patients
Once you enter your patient’s height and weight and choose the dosing frequency, the calculator will:
- Determine their BSA
- Calculate the minimum (9.9 g/m2/day) and maximum (13 g/m2/day) total daily dosages
- Calculate the minimum and maximum for each dose (ie, total divided among 3-6 doses)
- Indicate the recommended OLPRUVA dosing kit(s) for your patient
NOTE: Doses need to be rounded to match an available premeasured dosing envelope option; the calculator rounds down in order to ensure that patients do not exceed the maximum daily dosage.
This calculator uses the Du Bois method* to determine BSA.2 A number of other methods also exist for BSA determination.
*BSA = 0.007184 x Height0.725 x Weight0.425
Enter the patient’s height and weight, and select dosing frequency
Doses are available in the following strengths1:
OLPRUVA Packet(s) per Dosing Envelope‡
2 g

One 2-g packet
3 g

One 3-g packet
4 g

Two 2-g packets
5 g

One 2-g packet
One 3-g packet
6 g

Two 3-g packets
6.67 g

One 3-g packet
One 3.67-g packet
‡A Mix-Aid packet is included in each single-dose envelope.
OLPRUVA is conveniently supplied in kits that contain 90 pocket-sized, premeasured dosing envelopes (up to 30 days of treatment)1
- Eliminates the need for patients or caregivers to measure the medication before mixing it into the suspension
- Facilitates patient or caregiver transport of treatment when they’re on the go
Each dose (provided in 1 or 2 premeasured packets, depending on the patient’s dosage) is supplied inside an individual envelope that also contains a packet of Mix-Aid, which is mixed with 4 ounces (½ cup) of water to form the suspension.1
The patient will provide their own water, open drinking cup, and spoon.
No studies with OLPRUVA have been conducted in subjects with renal or hepatic impairment. Monitor ammonia levels, and in patients with hepatic impairment, it is recommended to start at the lowest dose that controls ammonia levels. Dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range.
Get the OLPRUVA
Prescription
Enrollment Form
Discover OLPRUVA
support and resources
for you and your
patients
Indication and Important Safety Information for OLPRUVA
OLPRUVA [ol proo vah] (sodium phenylbutyrate) for oral suspension
Indication
OLPRUVA is a nitrogen-binding agent indicated as adjunctive therapy to the standard of care, which includes dietary management, in the chronic management of adult and pediatric patients, weighing 20 kg or greater and with a body surface area (BSA) of 1.2 m2 or greater, with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS).
Limitation of Use
OLPRUVA is not indicated for the treatment of acute hyperammonemia, which can be a life-threatening medical emergency that requires rapidly acting interventions to reduce plasma ammonia levels.
Important Safety Information
Warnings and Precautions
Neurotoxicity of Phenylacetate: Increased exposure to phenylacetate, the major metabolite of OLPRUVA, may be associated with neurotoxicity in patients with UCDs. If neurotoxicity symptoms of vomiting, nausea, headache, somnolence, or confusion are present in the absence of high ammonia levels or other intercurrent illnesses, consider reducing the dose of OLPRUVA.
Hypokalemia: Renal excretion of phenylacetylglutamine may induce urinary loss of potassium. Monitor serum potassium during therapy and initiate appropriate treatment when necessary.
Conditions Associated with Edema: OLPRUVA contains 124 mg of sodium per gram of sodium phenylbutyrate, and the Mix-Aid contains 5 mg of sodium per packet. Calculate the total amount of sodium based on the patient’s body surface area. If a patient develops new-onset edema or worsening edema while on treatment, discontinue administration of sodium phenylbutyrate and initiate appropriate therapy.
Drug Interactions
Valproic acid, haloperidol, or corticosteroids may increase plasma ammonia levels. Monitor ammonia levels closely. Probenecid may inhibit renal excretion of metabolites of OLPRUVA including phenylacetate and phenylacetylglutamine; monitor for potential neurotoxicity.
Use in Specific Populations
No studies with OLPRUVA have been conducted in subjects with renal or hepatic impairment. Monitor ammonia levels and in patients with hepatic impairment, it is recommended to start at the lowest dose that controls ammonia levels. Dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range.
OLPRUVA should be used with caution in patients who are pregnant or planning to become pregnant. Report pregnancies to the manufacturer. There are no data on the presence of OLPRUVA in human milk, the effects on the breastfed infant, nor the effects on milk production. This should be considered when assessing the mother’s need for OLPRUVA.
Adverse Reactions
Most common adverse reactions (incidence ≥ 3%) are amenorrhea or menstrual dysfunction (irregular menstrual cycles), decreased appetite, body odor and bad taste or taste aversion.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
This information is not comprehensive.
For additional information, please see full Prescribing Information for OLPRUVA on OlpruvaHCP.com.
Indication and Important Safety Information for OLPRUVA
OLPRUVA [ol proo vah] (sodium phenylbutyrate) for oral suspension
Indication
OLPRUVA is a nitrogen-binding agent indicated as adjunctive therapy to the standard of care, which includes dietary management, in the chronic management of adult and pediatric patients, weighing 20 kg or greater and with a body surface area (BSA) of 1.2 m2 or greater, with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS).
Limitation of Use
OLPRUVA is not indicated for the treatment of acute hyperammonemia, which can be a life-threatening medical emergency that requires rapidly acting interventions to reduce plasma ammonia levels.
Important Safety Information
Warnings and Precautions
Neurotoxicity of Phenylacetate: Increased exposure to phenylacetate, the major metabolite of OLPRUVA, may be associated with neurotoxicity in patients with UCDs. If neurotoxicity symptoms of vomiting, nausea, headache, somnolence, or confusion are present in the absence of high ammonia levels or other intercurrent illnesses, consider reducing the dose of OLPRUVA.
Hypokalemia: Renal excretion of phenylacetylglutamine may induce urinary loss of potassium. Monitor serum potassium during therapy and initiate appropriate treatment when necessary.
Conditions Associated with Edema: OLPRUVA contains 124 mg of sodium per gram of sodium phenylbutyrate, and the Mix-Aid contains 5 mg of sodium per packet. Calculate the total amount of sodium based on the patient’s body surface area. If a patient develops new-onset edema or worsening edema while on treatment, discontinue administration of sodium phenylbutyrate and initiate appropriate therapy.
Drug Interactions
Valproic acid, haloperidol, or corticosteroids may increase plasma ammonia levels. Monitor ammonia levels closely. Probenecid may inhibit renal excretion of metabolites of OLPRUVA including phenylacetate and phenylacetylglutamine; monitor for potential neurotoxicity.
Use in Specific Populations
No studies with OLPRUVA have been conducted in subjects with renal or hepatic impairment. Monitor ammonia levels and in patients with hepatic impairment, it is recommended to start at the lowest dose that controls ammonia levels. Dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range.
OLPRUVA should be used with caution in patients who are pregnant or planning to become pregnant. Report pregnancies to the manufacturer. There are no data on the presence of OLPRUVA in human milk, the effects on the breastfed infant, nor the effects on milk production. This should be considered when assessing the mother’s need for OLPRUVA.
Adverse Reactions
Most common adverse reactions (incidence ≥ 3%) are amenorrhea or menstrual dysfunction (irregular menstrual cycles), decreased appetite, body odor and bad taste or taste aversion.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
This information is not comprehensive.
For additional information, please see full Prescribing Information for OLPRUVA on OlpruvaHCP.com.
References
- OLPRUVATM (sodium phenylbutyrate) for oral suspension. Prescribing information. Newton, MA: Acer Therapeutics Inc.
- Du Bois D, Du Bois EF. Clinical calorimetry tenth paper: a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863-871.