Dose preparation and administration
Learn how OLPRUVA® (sodium phenylbutyrate) for oral suspension is prepared and administered.
How OLPRUVA is prepared and administered
Here is a summary of the instructions for administering OLPRUVA. Direct your patients to the Instructions for Use or the Administration Video for detailed instructions on preparation and administration.1
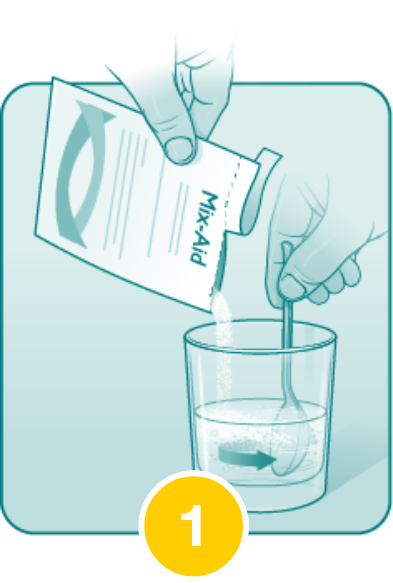
Pour the entire contents of the Mix-Aid packet into approximately 4 ounces of water in a cup and stir, forming a suspension.
- Instruct the patient NOT to drink the suspension yet
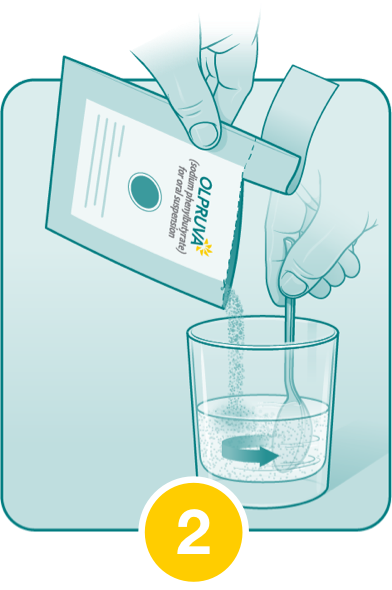
Pour the entire contents of the OLPRUVA packet(s) into the suspension and stir.
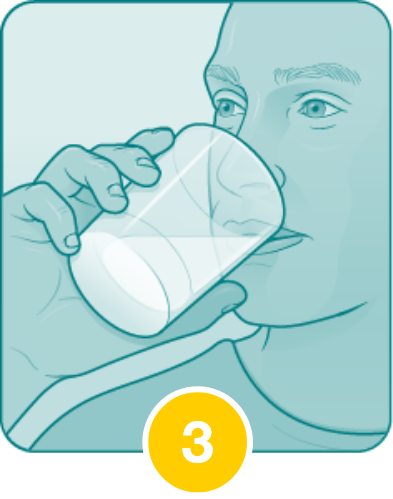
Drink the entire suspension within 5 minutes after stirring to minimize dissolution of coating.
- After 30 minutes, the suspension should be discarded
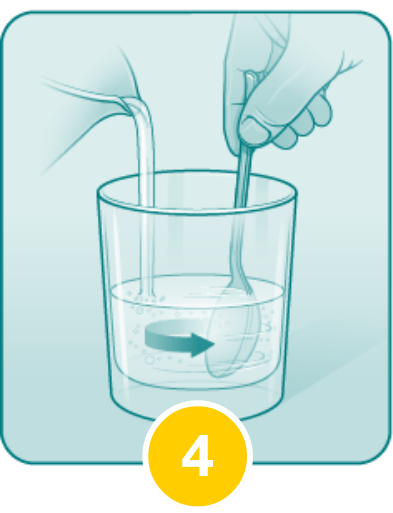
Pour another 4 ounces of water into the cup and drink in order to ensure that any OLPRUVA remaining in the cup is consumed.
See the Dosing page of this website for information on determining each patient’s appropriate dosage.
For oral administration only. Do not administer via gastrostomy or nasogastric tubes.
Patient Administration Video
Watch how patients should prepare and take or give a dose of OLPRUVA.
Learn about OLPRUVA
dosing options and
convenience
Get the OLPRUVA
Prescription
Enrollment Form
Indication and Important Safety Information for OLPRUVA
OLPRUVA [ol proo vah] (sodium phenylbutyrate) for oral suspension
Indication
OLPRUVA is a nitrogen-binding agent indicated as adjunctive therapy to the standard of care, which includes dietary management, in the chronic management of adult and pediatric patients, weighing 20 kg or greater and with a body surface area (BSA) of 1.2 m2 or greater, with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS).
Limitation of Use
OLPRUVA is not indicated for the treatment of acute hyperammonemia, which can be a life-threatening medical emergency that requires rapidly acting interventions to reduce plasma ammonia levels.
Important Safety Information
Warnings and Precautions
Neurotoxicity of Phenylacetate: Increased exposure to phenylacetate, the major metabolite of OLPRUVA, may be associated with neurotoxicity in patients with UCDs. If neurotoxicity symptoms of vomiting, nausea, headache, somnolence, or confusion are present in the absence of high ammonia levels or other intercurrent illnesses, consider reducing the dose of OLPRUVA.
Hypokalemia: Renal excretion of phenylacetylglutamine may induce urinary loss of potassium. Monitor serum potassium during therapy and initiate appropriate treatment when necessary.
Conditions Associated with Edema: OLPRUVA contains 124 mg of sodium per gram of sodium phenylbutyrate, and the Mix-Aid contains 5 mg of sodium per packet. Calculate the total amount of sodium based on the patient’s body surface area. If a patient develops new-onset edema or worsening edema while on treatment, discontinue administration of sodium phenylbutyrate and initiate appropriate therapy.
Drug Interactions
Valproic acid, haloperidol, or corticosteroids may increase plasma ammonia levels. Monitor ammonia levels closely. Probenecid may inhibit renal excretion of metabolites of OLPRUVA including phenylacetate and phenylacetylglutamine; monitor for potential neurotoxicity.
Use in Specific Populations
No studies with OLPRUVA have been conducted in subjects with renal or hepatic impairment. Monitor ammonia levels and in patients with hepatic impairment, it is recommended to start at the lowest dose that controls ammonia levels. Dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range.
OLPRUVA should be used with caution in patients who are pregnant or planning to become pregnant. Report pregnancies to the manufacturer. There are no data on the presence of OLPRUVA in human milk, the effects on the breastfed infant, nor the effects on milk production. This should be considered when assessing the mother’s need for OLPRUVA.
Adverse Reactions
Most common adverse reactions (incidence ≥ 3%) are amenorrhea or menstrual dysfunction (irregular menstrual cycles), decreased appetite, body odor and bad taste or taste aversion.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
This information is not comprehensive.
For additional information, please see full Prescribing Information for OLPRUVA on OlpruvaHCP.com.
Indication and Important Safety Information for OLPRUVA
OLPRUVA [ol proo vah] (sodium phenylbutyrate) for oral suspension
Indication
OLPRUVA is a nitrogen-binding agent indicated as adjunctive therapy to the standard of care, which includes dietary management, in the chronic management of adult and pediatric patients, weighing 20 kg or greater and with a body surface area (BSA) of 1.2 m2 or greater, with urea cycle disorders (UCDs) involving deficiencies of carbamylphosphate synthetase (CPS), ornithine transcarbamylase (OTC), or argininosuccinic acid synthetase (AS).
Limitation of Use
OLPRUVA is not indicated for the treatment of acute hyperammonemia, which can be a life-threatening medical emergency that requires rapidly acting interventions to reduce plasma ammonia levels.
Important Safety Information
Warnings and Precautions
Neurotoxicity of Phenylacetate: Increased exposure to phenylacetate, the major metabolite of OLPRUVA, may be associated with neurotoxicity in patients with UCDs. If neurotoxicity symptoms of vomiting, nausea, headache, somnolence, or confusion are present in the absence of high ammonia levels or other intercurrent illnesses, consider reducing the dose of OLPRUVA.
Hypokalemia: Renal excretion of phenylacetylglutamine may induce urinary loss of potassium. Monitor serum potassium during therapy and initiate appropriate treatment when necessary.
Conditions Associated with Edema: OLPRUVA contains 124 mg of sodium per gram of sodium phenylbutyrate, and the Mix-Aid contains 5 mg of sodium per packet. Calculate the total amount of sodium based on the patient’s body surface area. If a patient develops new-onset edema or worsening edema while on treatment, discontinue administration of sodium phenylbutyrate and initiate appropriate therapy.
Drug Interactions
Valproic acid, haloperidol, or corticosteroids may increase plasma ammonia levels. Monitor ammonia levels closely. Probenecid may inhibit renal excretion of metabolites of OLPRUVA including phenylacetate and phenylacetylglutamine; monitor for potential neurotoxicity.
Use in Specific Populations
No studies with OLPRUVA have been conducted in subjects with renal or hepatic impairment. Monitor ammonia levels and in patients with hepatic impairment, it is recommended to start at the lowest dose that controls ammonia levels. Dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range.
OLPRUVA should be used with caution in patients who are pregnant or planning to become pregnant. Report pregnancies to the manufacturer. There are no data on the presence of OLPRUVA in human milk, the effects on the breastfed infant, nor the effects on milk production. This should be considered when assessing the mother’s need for OLPRUVA.
Adverse Reactions
Most common adverse reactions (incidence ≥ 3%) are amenorrhea or menstrual dysfunction (irregular menstrual cycles), decreased appetite, body odor and bad taste or taste aversion.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
This information is not comprehensive.
For additional information, please see full Prescribing Information for OLPRUVA on OlpruvaHCP.com.
References
-
OLPRUVA™ (sodium phenylbutyrate) for oral suspension. Prescribing information. Newton, MA: Acer Therapeutics Inc.



